Research Projects
How do neurons continue to migrate in the infant brain?
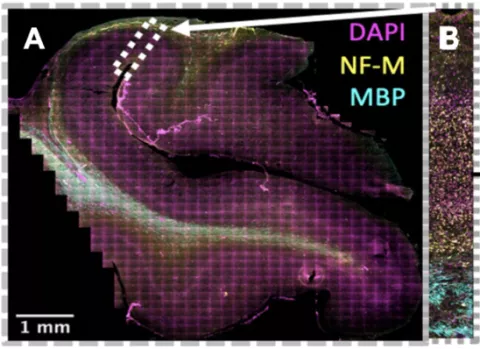
We have published evidence of robust neuronal migration in the postnatal human brain that contributes to the cortical network in the prefrontal cortex and cingulate during the first months of life. The presence of this migratory population demonstrates that the human cortex remains dynamic in the perinatal period, the time before and after birth, and raises the need to find methods to interrogate the functional importance of “late-migrating” neurons. Perinatal human cortical development, however, is incompletely represented in the lissencephalic (agyric) rodent. The long-term goal of my research is to understand the development of the gyrated neocortex during the perinatal period, the weeks immediately before and after birth, and how disruption during that time can lead to neurodevelopmental disorders (NDDs) such as ASD. Our central hypothesis is that neurons, especially inhibitory neurons, continue to travel perinatally to multiple cortical regions in the gyrencephalic (with infoldings) cortex, and that disruption of this migration contributes to abnormal behaviors. To investigate this, we use histological, transcriptomic and tissue culture techniques to study the early postnatal brain, focusing on the human brain and implementing gyrencephalic models, such as the piglet brain. These models will allow us to identify distinct properties of migratory neurons that persist late in neurodevelopment. We can then determine the cellular and molecular processes critical to perinatal cortical development and establish new ways to therapeutically influence these processes, even after birth. Figure shows young neurons, that express doublecortin (green) and polysialylated neural cell adhesion molecule, in the piglet cortex at postnatal day 1.
How do neural stem cells in the human hippocampus organize and maintain themselves?
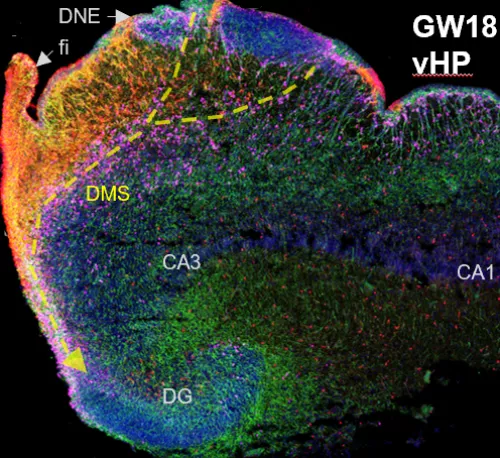
The hippocampus (HP) is a fundamental hub for processes such as memory and learning, and defects in its early formation can lead to severe cognitive deficiencies and neuropathological conditions, such as in epilepsy. The dentate gyrus (DG), a structure within the hippocampus, is considered the entryway for external hippocampal inputs and a central actor in internal HP circuitry. The mammalian DG also contains a subregion called the subgranular zone (SGZ), a cellular niche that maintains proliferation of neural progenitor cells (NPCs) throughout life. Understanding the normal development of the human DG is critical to defining the role of the HP in cognition and its vulnerability to disease. NPCs are the foundation that drive gliogenesis and neurogenesis (the production of new glia and neurons, respectively). However, the nature and development of NPCs in the human DG is largely unknown and the persistence of human adult neurogenesis remains an active debate in the field. The study of the gestational and early postnatal development of the human DG is therefore crucial to understanding the heterogeneity, structure, and dynamics of NPCs, as well as their persistence with age. Our studies seek to identify the mechanisms that regulate human NPC maintenance and how these normal processes could be affected in disease conditions, such as epilepsy. Delineating the regulation of DG neurogenesis in the human brain also has direct implications for the possibility of regeneration and neural repair after birth. The image shows the human hippocampus at 18 gestational weeks. It contains neural stem cells, (green), intermediate progenitor cells (pink), and astrocytes (red); cell nuclei are in blue.
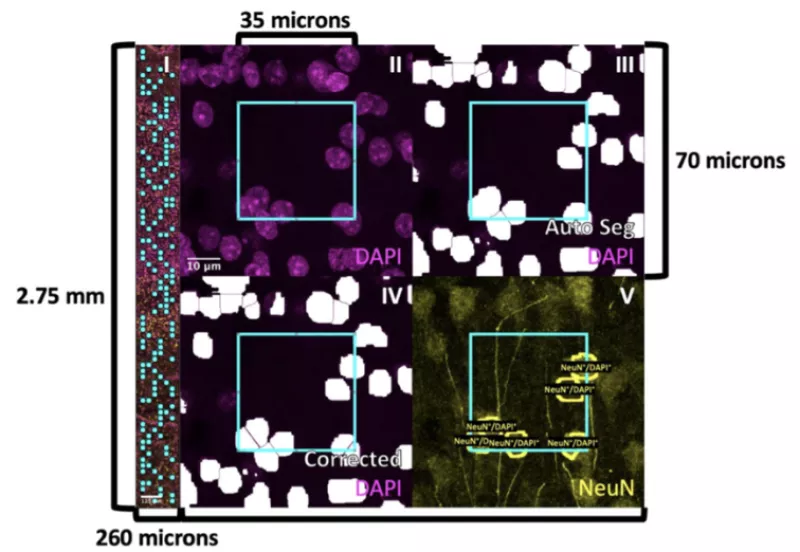
How does experience in infancy affect the cellular composition of human visual cortex?
As infants navigate the world outside of the womb for the first time, they begin to acquire cognitive and behavioral capabilities crucial to their survival, like the ability to recognize their parent’s faces. However, we have little knowledge how the cellular composition of brain regions like visual cortexis shaped by experience to perform these essential functional tasks. In this project, we are developing novel, automated methods to quantify the density and distribution of three major cell types in the brain—neurons, astrocytes, and oligodendrocytes—in primary visual cortex (calcarine sulcus) and associative brain regions responsible for face (mid fusiform sulcus) and scene (collateral sulcus) perception. We predict that these regions will display distinct cytoarchitectures and developmental timelines to accommodate their unique functional roles in visual processing. This research has a significant societal impact as strengthening our understanding of the typical development of human visual cortex will improve our ability to diagnose developmental diseases so that clinicians can intervene earlier and allow more babies to lead healthy, productive lives.