How does Experience in Infancy affect the Cellular Composition of Human Visual Cortex?
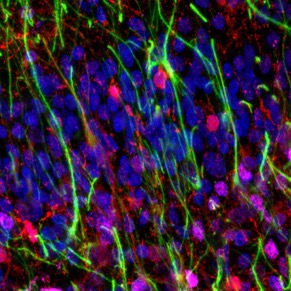
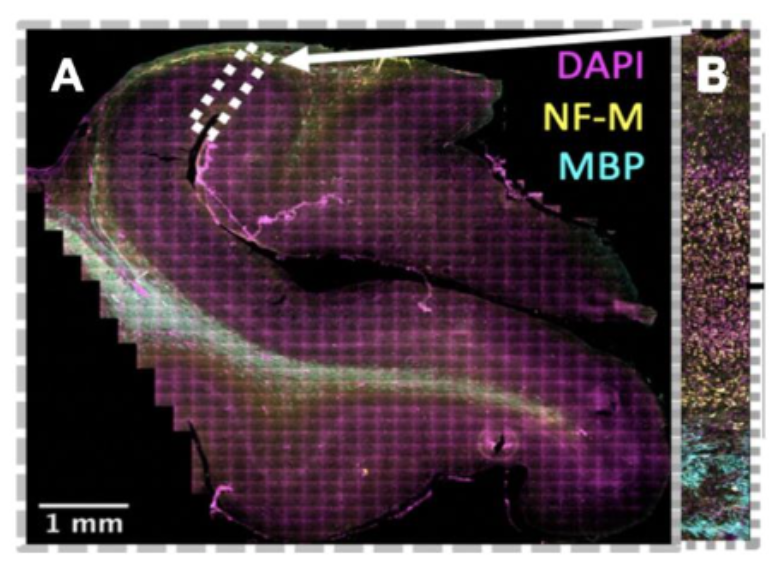
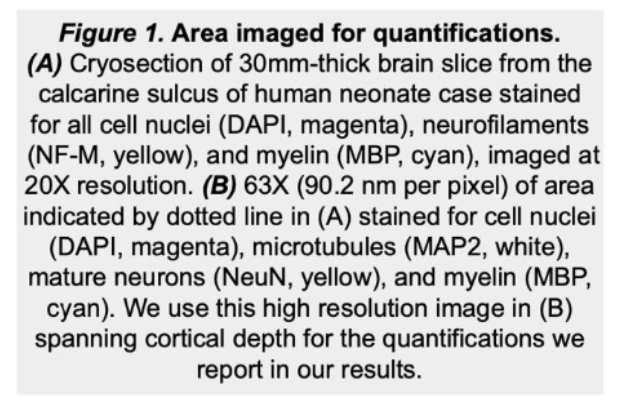
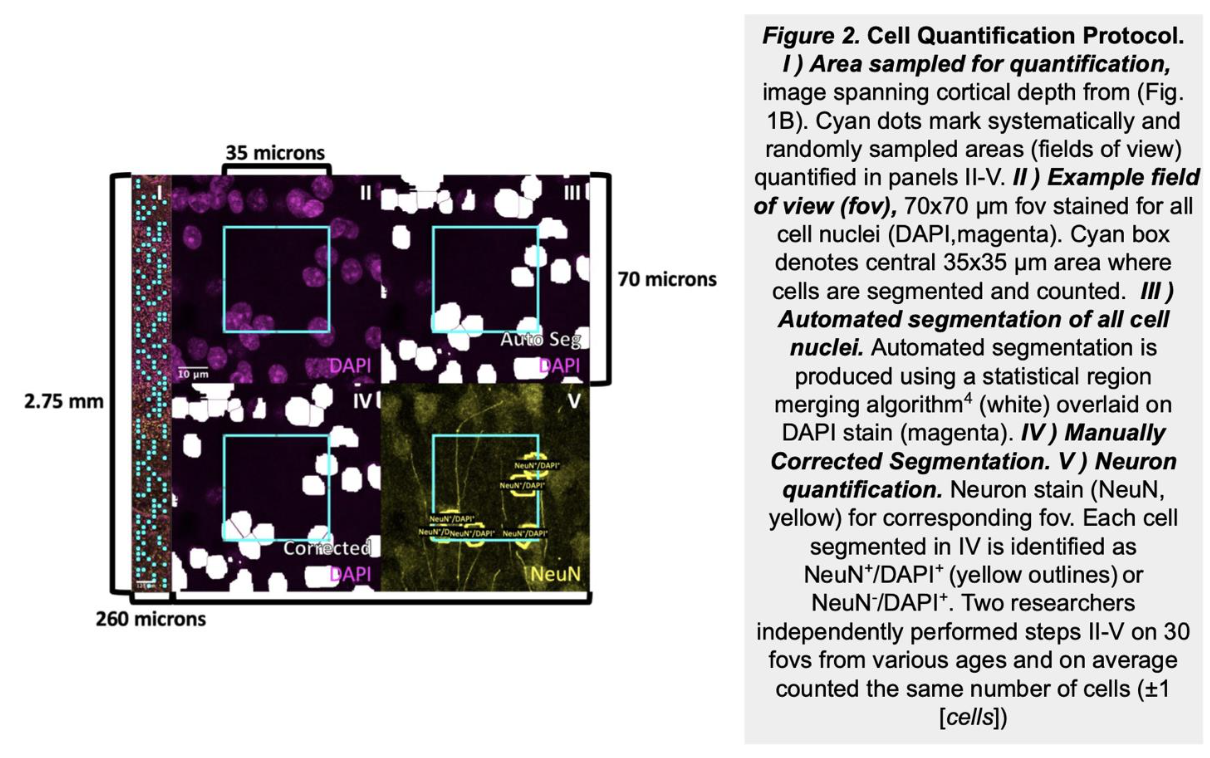
As infants navigate the world outside of the womb for the first time, they begin to acquire cognitive and behavioral capabilities crucial to their survival, like the ability to recognize their parent’s faces. However, we have little knowledge how the cellular composition of brain regions like visual cortexis shaped by experience to perform these essential functional tasks. In this project, we are developing novel, automated methods to quantify the density and distribution of three major cell types in the brain—neurons, astrocytes, and oligodendrocytes—in primary visual cortex (calcarine sulcus) and associative brain regions responsible for face (mid fusiform sulcus) and scene (collateral sulcus) perception. We predict that these regions will display distinct cytoarchitectures and developmental timelines to accommodate their unique functional roles in visual processing. This research has a significant societal impact as strengthening our understanding of the typical development of human visual cortex will improve our ability to diagnosis developmental diseases so that clinicians can intervene earlier and allow more babies to lead healthy, productive lives.
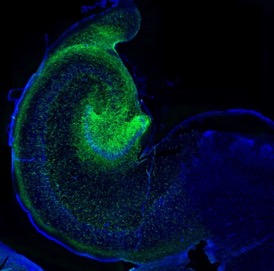
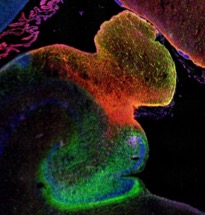
Developmental progression of NSCs in the human HP
The human hippocampus is widely known to be involved in important cognitive processes such as memory and learning, yet its embryonic and early postnatal development remains highly unknown. We work to unravel the mechanisms by which neural stem cells organize in the dentate gyrus and give rise to newborn neurons, as well as the extent of this process in time. We rely on histology and confocal-based microscopy to accurately define the spatial and morphological features of different cell types involved in the neurogenic cascade. We apply next-generation sequencing technologies in order to unravel signaling pathways that guide cell division and migration patterns in the developing hippocampus.
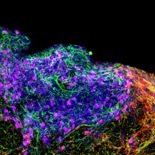
Cellular and molecular changes in the infant human HP after seizures
Temporal lobe epilepsy (TLE) affects hippocampus (HP) and adjacent brain regions, highly impairing cognitive abilities of an individual. Seizures taking place in early-life can trigger the onset of TLE at long-term. Although the HP bears no neurogenic capacity in the adulthood, radial glia cells, intermediate progenitors and immature neurons are present during development, including the early postnatal period. We propose that seizures alter hippocampal plasticity in the infant brain through unique signaling pathways that are absent in the adult. We evaluate transcriptomic changes after seizures through single cell RNA- and bulk RNA-sequencing and we will modulate the pathways using in-vitro slice cultures.